Company to open corporate headquarters in Minneapolis, MN
Minneapolis, MN – June 26, 2018 – Relievant Medsystems, a privately-held medical device company developing minimally-invasive solutions for chronic low back pain (CLBP), announced today the completion of a $58 million equity financing. The round was led by Endeavour Vision and included participation from new investor RK Mellon, as well as existing investors New Enterprise Associates, Canaan Ventures, Lightstone Ventures, and Emergent Ventures.
“We are pleased to have the support and confidence of this highly respected group of investors,” said Kevin Hykes, President and Chief Executive Officer of Relievant. “These new funds will facilitate the US commercial launch of the Intracept Procedure, providing a new, clinically-proven option to the more than five million indicated patients for whom today’s limited CLBP treatment options have proven ineffective.”
Relievant has developed the Intracept® Procedure, a minimally-invasive and clinically-proven approach for the treatment of CLBP and will use the new capital to commercialize the procedure in the United States. The funds will also support continued enrollment in the INTRACEPT study, the company’s second Level I prospective randomized controlled trial, which is being conducted at 20 sites in the US.
“Endeavour Vision is proud to partner with Relievant to support the launch of this important new therapy,” said Alexander Schmitz, who recently joined the Relievant board representing Endeavour Vision. “The ability to intervene early in the CLBP treatment continuum with the Intracept Procedure has the potential to significantly reduce pain and disability, decrease use of healthcare resources, and positively impact the opioid epidemic.”
The company also announced the opening of its corporate headquarters in Minneapolis, MN; it will maintain its existing R&D and operations facility in Sunnyvale, CA.
About Chronic Low Back Pain (CLBP)
Chronic low back pain is a widespread and often severely debilitating condition that is estimated to affect nearly 30 million people in the US, costing nearly $150 billion each year in medical treatment and lost productivity. Patients suffering from CLBP typically initiate treatment with conservative therapies such as activity modification, medications, bracing, physical therapy, manipulation and steroid injections. Existing therapies fail to provide adequate pain relief for approximately 80 percent of CLBP patients.
About the Intracept Procedure
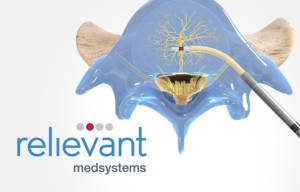
Relievant’s Intracept Procedure is a new, proven treatment option for the over five million indicated US patients who suffer from CLBP who have not responded to conservative therapies and who may not be candidates for surgery. The marketing clearance from FDA was supported by ground-breaking anatomic research that identified and demonstrated the role of the basivertebral nerve (BVN) in generating low back pain from the vertebral body endplates. The procedure uses a transpedicular, minimally-invasive approach to reach the BVN near the center of the vertebral body and uses radiofrequency energy to ablate the nerve; once ablated, these nerves no longer transmit pain signals. Spine surgeons and interventional pain specialists perform the Intracept Procedure under image guidance in the outpatient setting, with the entire procedure taking 60-90 minutes. Treated patients report nearly immediate relief of their back pain.
About Endeavour Vision
Endeavour Vision is an internationally-recognized investor in healthcare and technology companies. The team, which partners successful investment professionals with world-class industry veterans, has executed more than 75 investments within its focus verticals. Endeavour Vision is currently advising the Endeavour Medtech Growth Fund, a €250-million fund dedicated to helping medical technology companies in Europe and the US accelerate their global growth and market leadership. For more information, visit www.endeavourvision.com.
About Relievant Medsystems
Founded in 2006 and with offices in Minneapolis, MN, and Sunnyvale, CA, Relievant Medsystems is a privately held medical device company developing new solutions to improve the quality of life for millions of patients suffering from CLBP. FDA has cleared Relievant’s Intracept System for the following Indications for Use: The Intracept Intraosseous Nerve Ablation System is intended to be used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain of at least 6 months duration that has not responded to at least six months of conservative care, and is also accompanied by either Type 1 or Type 2 Modic changes on an MRI.
As with any surgical procedure, there are risks and considerations associated with the Intracept Procedure. Please visit www.relievant.com for a discussion of the risks, contraindications, warnings, precautions and a summary of the pivotal clinical trial data on the device.
Contact
Chris Geyen
Relievant Medsystems
(650) 368-1000
Originally posted by Relievant





